XRF Spectrometry Data Method
XRF Spectrometry can use either Empirical Methods (calibration curves using standards similar in property to the unknown) or Fundamental Parameters (FP) to arrive at quantitative elemental analysis. The GEMORO XRF Gold and Alloy Analyzeruses the superior Fundamental Parameters because it allows elemental analysis to be performed without standards or calibration curves, which is much more preferred. This enables the analyst to use the system immediately, without having to spend additional time setting up individual calibration curves for the various elements and materials of interest. FP, accompanied by stored libraries of known materials, determines not only the elemental composition of an unknown material quickly and easily, but can identify unknown materials as well.
More on How XRF works:
XRF works by striking a sample with an X-ray beam from an X-ray tube, causing characteristic X-rays to fluoresce from each element in the sample. A detector measures the energy and intensity (number of X-rays per second at a specific energy) of each X-ray, which is transformed into an elemental concentration using either a non-standard technique such as fundamental parameters or user-generated calibration curves. The presence of an element is identified by the element’s characteristic X-ray emission wavelength or energy. The amount of an element present is quantified by measuring the intensity of that element’s characteristic X-ray emission.
The Atomic Level
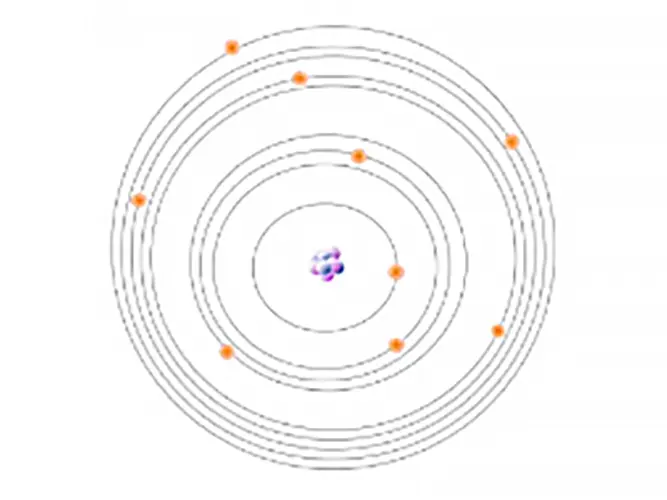
All atoms have a mixed number of electrons. These electrons are arranged in orbitals around the nucleus. Energy Dispersive XRF (EDXRF) typically captures activity in the first three electron orbitals, the K, L, and M lines.
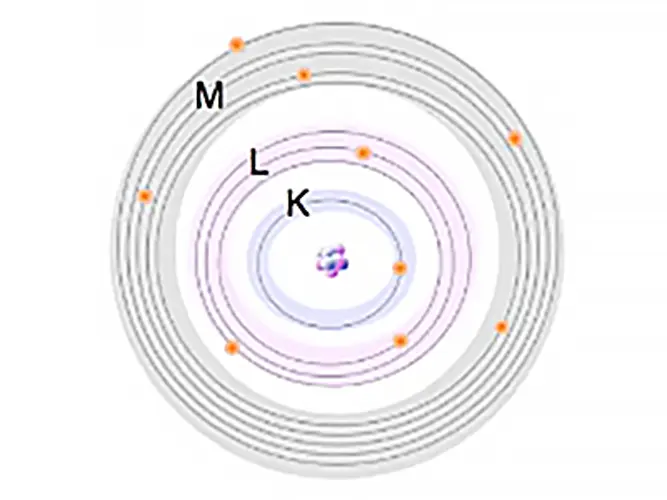
These electrons are arranged in orbitals around the nucleus. Energy Dispersive XRF (EDXRF) typically captures activity in the first three electron orbitals, the K, L, and M lines.
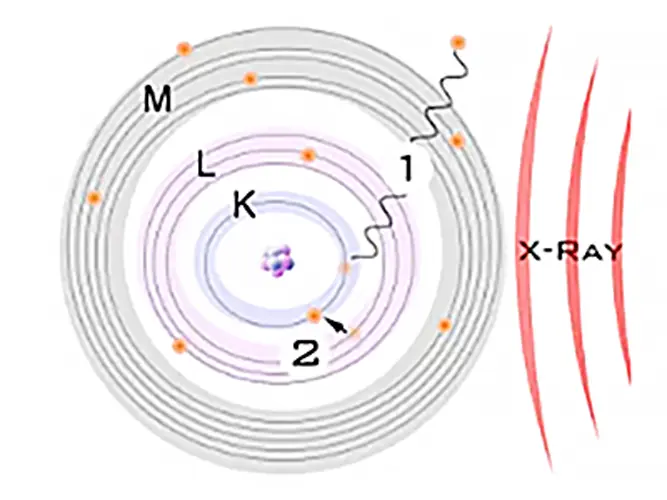
The primary photons from the X-ray tube have high enough energy that they knock electrons out of the innermost orbitals, creating a vacancy (1). An electron from an outer orbital will move into the newly vacant space at the inner orbital to regain stability within the atom (2).
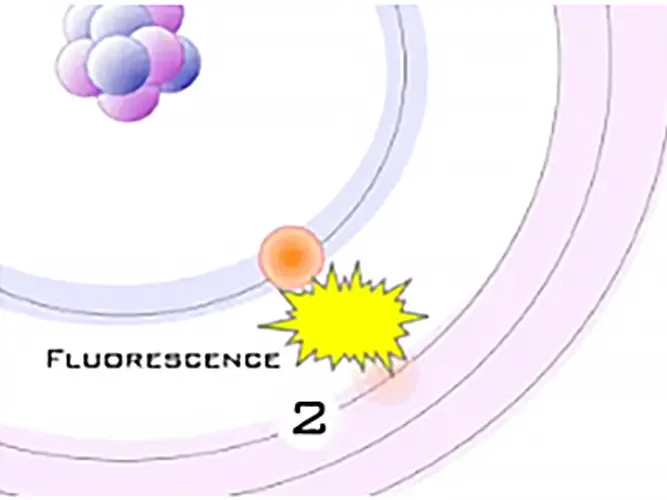
As the electron from the outer orbital moves into the inner orbital, it releases energy in the form of a secondary X-ray photon. This energy release is known as fluorescence. All elements produce fluorescence “characteristic” to themselves. Each element’s fluorescence is unique to itself.
X-RAY
Sample Material
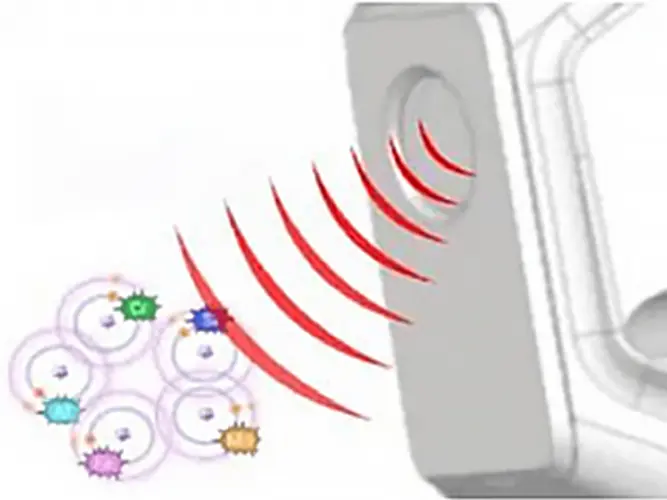
High-energy primary X-ray photons are emitted from an X-ray tube and strike the sample.
DETECTOR
Sample Material
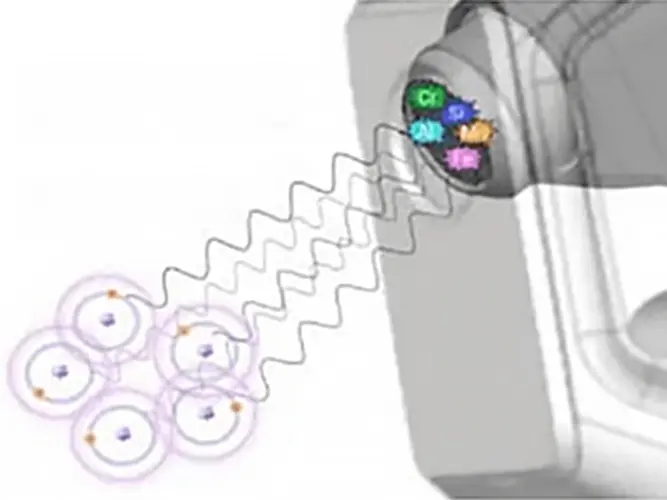
The fluorescent energy is transferred to a detector, where it is absorbed and transferred into an electrical signal and then into a number (digitized).
While XRF analyzing technology is very precise, please recognize that this technology measures the elements present on the surface (AKA a “surface test”), and it should not be considered as a substitute for a fire assay of a core sample.

























